
Worldwide, diabetes is a major public health problem affecting a large number of individuals. The global prevalence of diabetes in adults has been increasing over recent decades. The total number of people with diabetes is projected to increase from 483 million to 700 million1. India has currently 77 million people with diabetes while 80 million have pre-diabetes.
Despite advancements in both pharmaceutical and technological treatment options, diabetes is still a big concern. Type 2 diabetes has long been considered a chronic incurable or irreversible disorder. So far, the major focus has been on control of blood sugars and delaying the progression to a stage of complications. Recently, a shift in trying to reverse diabetes completely has occurred.
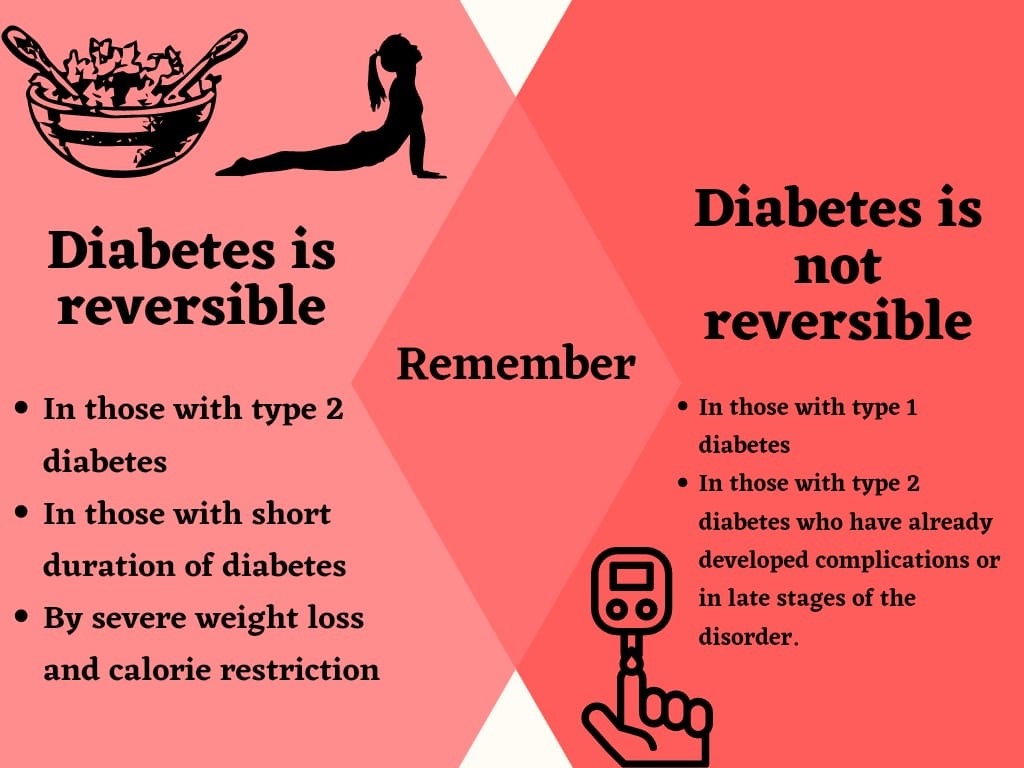
There is no cure for diabetes as such, but the disorder can go into a remission phase. Remission is when blood glucose levels return to within the normal range even without the use of medications. However, this does not mean that diabetes has got cured completely. The body may not show any signs of diabetes but technically it is still present as the genetic and some biochemical defects still remain. When a person is in an early stage of diabetes or pre-diabetic stage, reversal is potentially achievable and relatively easy.
There is a subtle difference between ‘reversal’ and ‘remission’. Remission implies normal HbA1c without the need for any anti-diabetic medications, whereas, ‘Reversal’ could also mean a reduction in the number/dosage of anti-diabetic medications. For example, a reversal from an insulin-requiring stage to a non-insulin-requiring stage of the disorder. Reversal of diabetes usually occurs due to an improvement in insulin sensitivity. The American Diabetes Association (ADA) defines the following three mutually exclusive states of remission: partial remission, which is subdiabetic hyperglycemia of at least 1 year (A1C level not diagnostic of diabetes [5.7–6.4%; 39–46 mmol/mol], fasting glucose level 100–125 mg/dL [5.6–6.9 mmol/L]); complete remission, which is normoglycemia of at least 1 year (A1C level in the normal range [<5.7%; <39 mmol/mol], fasting glucose <100 mg/dL [5.6 mmol/L]); and prolonged remission (or “cure”), complete remission of at least 5 years2.
What causes reversal or remission?
Weight reduction is the key behind both remission and reversal of diabetes. Studies have shown that both are possible through bariatric surgery, a very low-calorie diet, carbohydrate-restricted diet and exercise. There is a continuous relationship between weight loss and the consequent improvement in glycemic levels and the probability of T2DM remission. The results of the Diabetes Remission Clinical Trial (DiRECT), in which lifestyle measures consisting primarily of stringent dietary management and increased physical activity were implemented, provide proof for this. In the DiRECT trial, there was an increasing probability of T2DM remission across categories of increasing absolute weight loss.3
Remission or reversal of diabetes is mainly achieved by two types of diets, very-low-calorie diets (VLCD) and low carbohydrate diets (LCD). We will discuss both in greater detail.
Very low-calorie diets:
Some studies show that a drastic reduction in calorie intake can reverse Type 2 diabetes, even in people who have had diabetes for over 10 years4.
If diabetes is in its initial stages, fasting blood glucose can become normal within a few days of a very low-calorie diet (VLCD). Drastic decrease of fat in the liver and improvement in insulin sensitivity are the major reasons for this. In 8 weeks, beta-cell (cells responsible for synthesising and secreting insulin) function returns to normal as well. If these levels are maintained, diabetes can be reversed.
Several studies have shown that blood sugars can be controlled well in patients with type 2 DM by calorie restriction using a very low-calorie diet5. In patients with severe obesity (body mass index [BMI] ~30 kg/m2). An improvement in hepatic insulin sensitivity was quickly observed after only a week of VLCD, while the insulin secretion was restored later (by 8 weeks)7. In patients with extreme obesity (BMI ~45 kg/m2), however, only an improvement in insulin secretion was observed with no changes in hepatic insulin sensitivity8. The rate of diabetes remission appeared to be higher among those who lost more weight9. Collectively, these studies suggest that the underlying patho-physiologic defects of type 2 DM can be reversed by VLCD.
In a study by Bauman et al., a low-calorie diet of 900 kcal, including 115 g of protein, led to significant improvement in glycemic control that was mainly attributed to improvements in insulin sensitivity10. Furthermore, a study conducted in obese T2D patients found that LCD and gastric bypass surgery were equally effective in achieving weight loss and improving glucose and HbA1c levels in the short term11. Weight loss, however, persisted in the diet-treated patients only for the first three months, indicating difficulty with long-term maintenance13. Lifestyle intervention with severe energy restriction may have some detrimental effect on the body composition and physiology, which may be a concern for long-term health. Adherence to the diet and maintenance of lost weight is the challenging factors for long-term remission or reversal of diabetes.
The low carbohydrate diets:
Before insulin was discovered in 1921, low carbohydrate (LC) diets were the standard of care for diabetes12. Patients are instructed to carefully restrict dietary `carbohydrates, eat protein in moderation, and consume enough dietary fats. LC diets have been shown to be more effective than restricting overall daily calorie intake. A study comparing an ad libitum (non-calorie restricted) very low carbohydrate (<20g total) diet to an energy-restricted low-glycemic diet in patients with T2D found a greater reduction in HbA1c, weight, and insulin levels in the low carbohydrate arm13.
LC diets focus more on macronutrient changes than on calorie restriction. Low-carbohydrate diets restrict the total carbohydrates to less than 130 grams per day. A very low-carbohydrate or ketogenic diet usually restricts total carbohydrates to as low as 20–30 grams per day. Protein consumption is not usually modified and hence the remaining energy needs are met by fat, which automatically means that LC diets are high-fat diets. The carbohydrate sources are primarily non-starchy vegetables with some nuts, dairy, and limited fruit14.
Based upon a recent systematic review of LC diets, it appears that the greatest improvements in glycemic control and greatest medication reductions have been associated with the lowest carbohydrate intake15. However, the side effects are that the diets are unbalanced and the blood lipids tend to go up drastically because of the high fat intake.
Keto diets are very similar to very low carbohydrate diets. When blood sugars are under very good control, the number and dosage of anti-diabetic medicines can be reduced and eventually withdrawn in many cases. Medications that rapidly reduce blood sugar need to be stopped, for example, insulin and sulfonylureas (sometimes in as little as 2 days to 2 weeks). Other medications like SGLT-2 inhibitors, DPP-4 inhibitors, GLP-1 receptor agonists, and metformin can be stopped as long as normal blood sugar levels are maintained.

Exercise:
Regular exercise should be encouraged for better control of blood sugars and reduction of cardiovascular risk. Muscles use more glucose during exercise than at rest. Exercising regularly is necessary for the successful reversal of type 2 diabetes as it helps the body to become more sensitive to its insulin. Exercise when combined with a healthy diet can reduce the demand for insulin in the body and therefore help reverse diabetes.
A study published in 2015 showed that 67% of participants who were able to achieve partial remission of their type 2 diabetes have taken part in a six-month diet and exercise program, kindly note however that the participants in this study were newly diagnosed with type 2 diabetes18.
Bariatric surgery:
One of the interventions, now widely used to treat severe obesity, is Bariatric Surgery (Weight Loss Surgery). Bariatric surgery could be appropriate for those with a body mass index (BMI) of over 35 with no co-morbidities or for those with a BMI of over 30 with significant co-morbidities like hypertension or dyslipidemia. Weight loss after surgery is more likely to be maintained in the long term than with lifestyle measures. Several studies have reported a decrease in mortality and severity of several medical conditions after bariatric surgery17. Bariatric surgery is effective in not only reducing weight but also results in resolution or significant improvement in diabetes including a total remission of diabetes in up to 70% of individuals for up to 6 years. Surgery can potentially reverse diabetes and as well as reduce high blood pressure, cholesterol and prevent cardiovascular disease complications in the long run17.
However, some patients still experience weight regain despite bariatric surgery. Effective dietary interventions are imperative in these scenarios.
Undoubtedly, bariatric surgery is the most effective method for overall efficacy and prolonged remission. However, surgical complications, treatment cost and the fact lifestyle modification is still needed after surgeries are still major concerns. While both the LCD and LC dietary approaches are convincing for reversing diabetes in the short term (up to two years), long-term maintenance of diabetes remission is still unproven. There are limited available data supporting long-term maintenance of weight loss and its associated glycemic improvements in response to LCD; likewise, adhering to the low carbohydrate diet for a prolonged period of time will remain challenging without the development of proper patient education and optimal support for long-term behavioral change.
Determining the appropriate method of educational support may be the key to the overall success with reversal of diabetes. Longer-term studies are needed to determine the long-term sustainability of diabetes reversal/ remission. Finally, it must be emphasized that whenever one talks of diabetes remission or reversal, we are talking about type 2 diabetes. Type 1 diabetes is a completely different disease and attempts to stop insulin or reverse type 1 diabetes can be dangerous and can even be fatal, at this time.
References:
- King H, Aubert RE, Herman WH.Diabetes Care. 1998 Sep;21(9):1414-31. doi: 10.2337/diacare.21.9.1414.PMID: 9727886
- Buse J.B., Caprio S., Cefalu W.T., Ceriello A., Del Prato S., Inzucchi S.E., McLaughlin S., Phillips G.L., II, Robertson R.P., Rubino F., et al. How do we define cure of diabetes? Diabetes Care. 2009;32:2133–2135. doi: 10.2337/dc09-9036
- Lean M.E., Leslie W.S., Barnes A.C., Brosnahan N., Thom G., McCombie L., Peters C., Zhyzhneuskaya S., Al-Mrabeh A., Hollingsworth K.G., et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. 2018;391:541–551. doi: 10.1016/S0140-6736(17)33102-1
- Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet2017;391:541–51. [PubMed] [Google Scholar]
- Malandrucco et al., 2012; Wing et al., 1994). Gregg, E. W. , Chen, H. , Wagenknecht, L. E. , Clark, J. M. , Delahanty, L. M. , Bantle, J. , … Look AHEAD Research Group . (2012). Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA, 308(23), 2489–2496. 10.1001/jama.2012.67929
- ). Lim, E. L. , Hollingsworth, K. G. , Aribisala, B. S. , Chen, M. J. , Mathers, J. C. , & Taylor, R. (2011). Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia, 54(10), 2506–2514. 10.1007/s00125-011-2204-7
- Malandrucco, I. , Pasqualetti, P. , Giordani, I. , Manfellotto, D. , De Marco, F. , Alegiani, F. , … Frontoni, S. (2012). Very‐low‐calorie diet: A quick therapeutic tool to improve beta cell function in morbidly obese patients with type 2 diabetes. American Journal of Clinical Nutrition, 95(3), 609–613. 10.3945/ajcn.111.023697
- Steven, S. , Hollingsworth, K. G. , Al‐Mrabeh, A. , Avery, L. , Aribisala, B. , Caslake, M. , Taylor, R. (2016). Very low‐calorie diet and 6 months of weight stability in type 2 diabetes: Pathophysiological changes in responders and nonresponders. Diabetes Care, 39(5), 808–815. 10.2337/dc15-1942.
- Bauman W.A., Schwartz E., Rose H.G., Eisenstein H.N., Johnson D.W. Early and long term effects of acute caloric deprivation in obese diabetic patients. J. Med. 1988;85:38–46. doi: 10.1016/0002-9343(88)90500-1.
- Hughes T.A., Gwynne J.T., Switzer B.R., Herbst C., White G. Effects of caloric restriction and weight loss on glycemic control, insulin release and resistance, and atherosclerotic risk in obese patients with type II diabetes mellitus. J. Med. 1984;77:7–17. doi: 10.1016/0002-9343(84)90429-7.
- Sjostrom L., Lindroos A.K., Peltonen M., Torgerson J., Bouchard C., Carlsson B., Dahlgren S., Larsson B., Narbro K., Sjostrom D., et al. Lifestyle, diabetes and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622.
- Westman, EC.; Yancy, WS.; Humphreys, M. Dietary treatment of diabetes mellitus in the pre-insulin era (1914-1922). Perspectives in Biology and Medicine. 2006, 49, 77-83.
- Westman EC, Yancy WS, Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab. 2008, 19:36. doi:10.1186/1743-7075-5-36.
- Westman E.C., Feinman R.D., Mavropoulos J.C., Vernon M.C., Volek J.S., Wortman J.A., Yancy W.S., Phinney S.D. Low carbohydrate nutrition and metabolism. J. Clin. Nutr. 2007;86:276–284. doi: 10.1093/ajcn/86.2.276.
- Elhayany A., Lustman A., Abel R., Attal-Singer J., Vinker S. A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: A 1-year prospective randomized intervention study. Diabetes Obes. Metab. 2010;12:204–209. doi: 10.1111/j.1463-1326.2009.01151.x.
- Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, et al. The Indian Council of Medical Research-India Diabetes (ICMR-INDIAB) study: methodological details. J Diabetes Sci Technol. 2011;5:906–14.
- Chandru S, Pramodkumar TA, Pradeepa R, Jebarani S, Prasad YM, Praveen RP, Sathish Babu J, Anjana RM, Mohan V. Impact of bariatric surgery on body composition and metabolism among obese Asian Indians with prediabetes and diabetes. J Diabetol 2021;12:208-17




